Biosynthesis of Riboflavin (Flavocoenzymes)
Flavocoenzymes are essential cofactors for
catalysis of a wide variety of redox reactions. Moreover, they are
involved in numerous other physiological processes involving light sensing,
bioluminescence, circadian time-keeping and DNA repair.
Vitamin B2 (riboflavin) is the universal precursor of flavocoenzymes. Riboflavin is biosynthesized in plants and in
many bacteria. Vegetables and milk are major sources of the vitamin in
human nutrition. Ruminants can derive vitamin B2 from their intestinal
flora. The daily recommended allowance for vitamin B2 is 1.8 mg. Although
the flavocoenzymes are absolutely indispensable in all cellular organisms,
symptoms of riboflavin deficiency are rarely observed in man. However,
latent riboflavin deficiency may be relatively common, especially in women
and adolescents – especially in developmental countries.
The compound is manufactured in relatively
large quantity (about 4000 metric tons per year) for use as a vitamin in
human and animal nutrition and as a colorant, and biotechnological aspects
were an important driving form for studies on its biosynthesis which
extend over a period of more than five decades. In fact, the manufacture
of the vitamin by fermentation has by now essentially replaced chemical
synthesis.
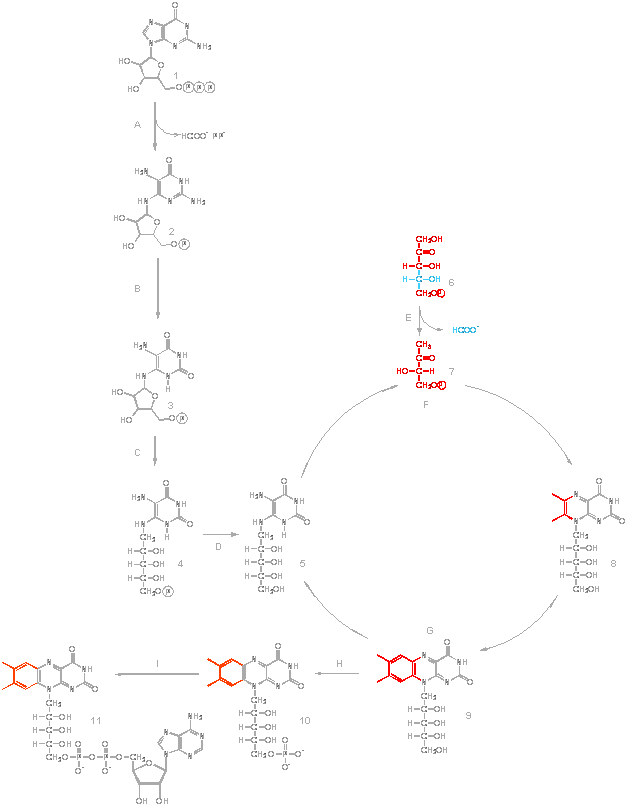
The
biosynthesis of one riboflavin molecule requires one molecule of GTP (1)
and two molecules of ribulose 5-phosphate (6). The imidazole ring of GTP
is hydrolytically opened by the GTP cyclohydrolase II (A), yielding a
4,5-diaminopyrimidine that is converted to
5-amino-6-ribitylamino-2,4(1H,3H)-pyrimidinedione (4)) by a sequence of
deamination (B), side chain reduction (C), and dephosphorylation (D).
Condensation of 5-amino-6-ribitylamino-2,4(1H,3H)-pyrimidinedione (5) with
3,4-dihydroxy-2-butanone 4-phosphate (7) obtained from ribulose
5-phosphate affords 6,7-dimethyl-8-ribityllumazine (8). This reaction is
catalysed by the 6,7-dimethyl-8-ribityllumazine synthase (F). Dismutation
of the lumazine derivative (riboflavin synthase; G) yields riboflavin (9)
and 5-amino-6-ribitylamino-2,4(1H,3H)-pyrimidinedione (5), which is
recycled in the biosynthetic pathway.
Flavoenzymes contain flavin nucleotides as redox cofactors, either
riboflavin monophosphate (FMN; 10) or flavin adenine dinucleotide (FAD;
11). All organisms are able to convert the precursor, riboflavin, into the
active flavin nucleotide cofactors. The formation of FAD depends on the
sequential utilisation of two molecules of ATP in reactions that first
involve the phosphorylation of riboflavin to form FMN by riboflavin kinase
(H) and then the adenylation of the latter to form FAD, a reaction
catalysed by FAD synthetase (I).
Enzymes from plants and microorganisms (yeast, eubacteria, archaea)
involved in this pathway, their reaction mechanisms and their structures
are mainly topics in the riboflavin division. Among this, both biophysical
and biochemical methods are employed.
Structure and function of lumazine protein
A number of fluorescent proteins (e.g.
lumazine protein, yellow fluorescent protein, blue fluorescent protein)
have been found to serve as optical transponders in luminescent
photobacteria. They are brought to an excited state by Förster transfer
from bacterial luciferase. Their overall contribution to bioluminescence
is an increase in quantum yield and a shift of the emission spectrum.
A non-covalently bound molecule of 6,7-dimethyl-8-ribityllumazine serves
as fluorophore of the lumazine protein, the emitter protein of
Photobacterium leiognathi.
Modulation of the binding site by site directed mutagenesis in conjunction with the spectroscopic techniques (multinuclear NMR, EPR, ENDOR, fluorescence spectroscopy and X-ray structure analysis) is expected to provide structural as well as dynamic information on protein-ligand interaction and its role for luminescence amplification by enhanced fluorescence quantum yield.